South Korea Covid Vaccine NFCS
Introduction
South Korea had an existing no-fault compensation scheme for vaccines created 29 December 2009, and which incorporated covid-19 vaccines from 10 February 2021.
This scheme was created under national legislation. It is governed by Article 71 of the Infectious Disease Control and Prevention Act (Act No. 17067, March 4 2020) and the Enforcement Decree of the Infections Disease Control and Prevention Act (Presidential Decree No. 28070, May 29 2017).
It is administered by a combination of local and national government officials as set out in Article 31 of the Enforcement Decree. The pathways for claims are set out briefly below:-
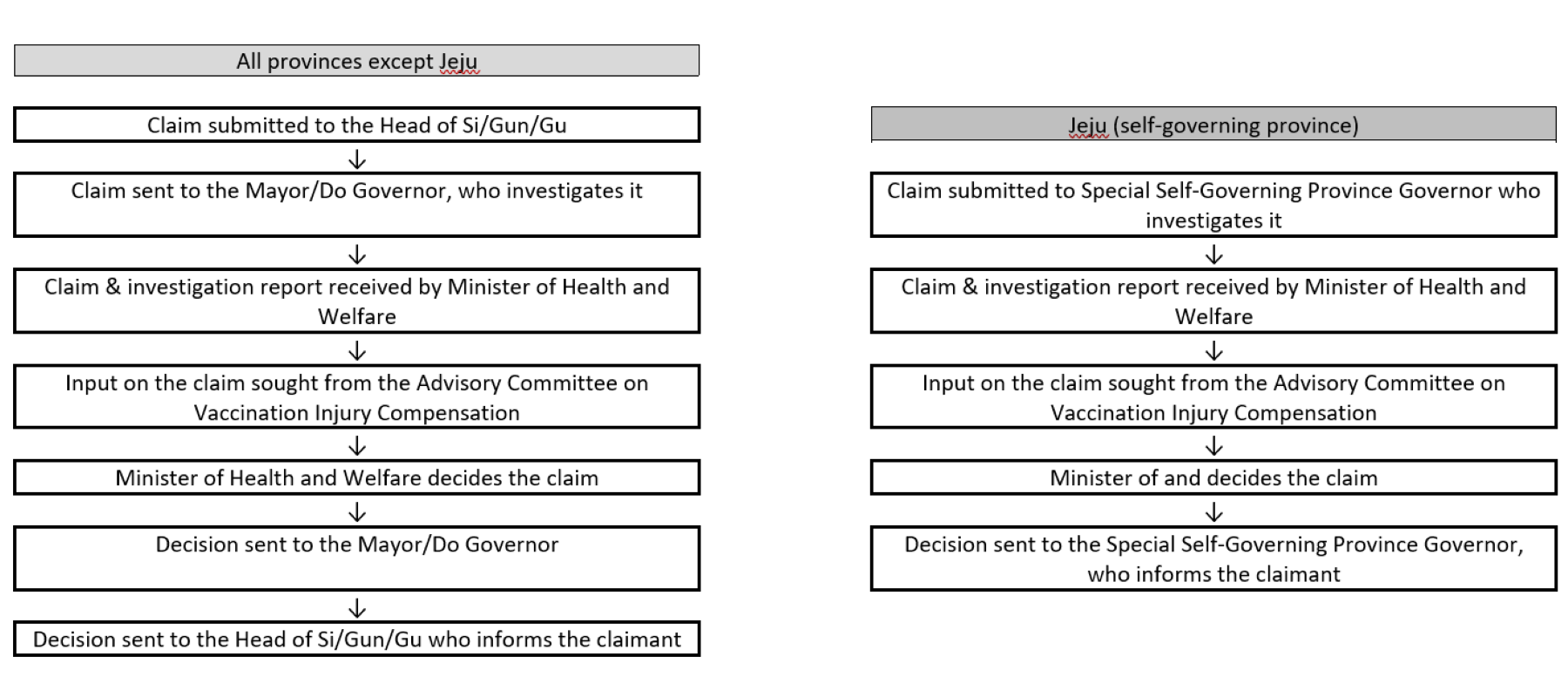
Responsibility for the scheme and the compensation decisions rests with the Minister of Health and Welfare. Under Article 32 of the Decree these functions are delegated to the Director of the Korea Disease Control and Prevention Agency (KDCA). KDCA is a public body under the Ministry of Health and Welfare. Press reports, for example see here and here indicate that on 19 July 2021 KDCA announced a change of administrator from themselves to a newly created Compensation & Support Center for Covid-19 Vaccine Injury. We have not been able to find any further information on the Compensation & Support Center for Covid-19 Vaccine Injury.
The funding for the scheme comes from central government.
Vaccines Covered
This NFCS covers vaccines purchased by the Government and administered at public Health Centres. Both mandatory vaccines (Art 24 of the Act) and special (voluntary) vaccines (Art 25 of the Act) are covered. This NFCS covers nationally approved vaccines, approved for emergency use and standard approvals.
Injuries Covered
This NFCS covers both temporary and permanent injuries.
Under this NFCS any injury is potentially covered.
Under this NFCS only eligible injuries are covered. Eligible individuals are those who have contracted a disease, become disabled or died due to vaccination (Article 71 of the Act).
Charges for making a claim
There is no charge for making a claim under this scheme.
Claimants
Under this scheme the following categories of individuals are permitted to make a claim.
- Live vaccine recipient/their authorised representative
- The estate/representative of a deceased vaccine recipient
This scheme does not specify whether the claimant is allowed to nominate a legal representative to make their claim. It is not clear if funding for legal representation is provided by the scheme.
Losses covered
This scheme pays the following
| Live Vaccine Recipient | Dependants of Vaccine Recipient | Estate of Deceased Vaccine Recipient |
| Only Eligible economic losses are compensated | No Compensation is provided specifically for dependants | Only Eligible economic losses are compensated |
Article 29 of the Decree sets out the compensation available. The following categories of benefits and reimbursements are available.
Living vaccine recipients
- Medical expenses – the balance of medical expenses for treating the vaccine injury, less any amount due to an insurer or medical care fund.
- Nursing expenses – flat rate of KRW 50,000 per day – only paid for inpatient treatment
- Disability compensation – a fixed percentage of the monthly minimum wage x 240 months
If a payment is made for disability compensation then no further medical expenses are paid.
The monthly minimum wage is set at the minimum wage when the injury occurred as defined in the Minimum Wage Act. It is updated regularly, in February 2023 the monthly minimum wage was KRW 2,010,580.
| Disability | Lump Sum compensation |
| Grade 1 | 100 % of the monthly minimum wage x 240 months |
| Grade 2 | 85 % of the monthly minimum wage x 240 months |
| Grade 3 | 70 % of the monthly minimum wage x 240 months |
| Grade 4 | 55 % of the monthly minimum wage x 240 months |
| Grade 5 | 40 % of the monthly minimum wage x 240 months |
| Grade 6 | 25 % of the monthly minimum wage x 240 months |
Deceased Vaccine recipients
- Death Benefits – monthly minimum wage x 240 months
- Funeral expenses – KRW 300,000
Payments consist of a lump sum payment.
Funeral expenses of KRW 300,000 are available under this NFCS.
Compensation for medical expenses under this scheme is fully individualised.
Death benefits and Disability Compensation under this scheme are calculated on an individual basis using tariffs to assist with quantification. These payments are a form of loss of earnings.
Compensation for nursing expenses and funeral expenses under this scheme are a fixed sum amount of and KRW 50,000 per day and KRW 300,000 respectively.
Compensation under this scheme is not capped.
Time limits for claims
The scheme does not set a time limit between vaccination and the adverse event occurring.
A claim under the scheme must be brought within five years from the date of the injury, the diagnosis or the death as applicable.
Evaluating claims – standard of proof required
Under Article 9 of the Infectious Disease Control and Prevention Act the Infectious Diseases Committee ‘the Committee’ will be established by the Ministry of Health and Welfare, with the Minister appointing the Committee members. The Committee shall comprise no more than 30 members, including a Chair and Vice-Chair. Article 10 of the Act specifies that the Chair will be the Director of KDCA. The Chair appoints the Vice-Chair. Committee members will be chosen from the following categories:-
- Public officials in charge of duties of preventing and controlling infectious diseases;
- Medical personnel specializing in infectious diseases or infectious disease control;
- Persons with expertise related to infectious diseases;
- Persons recommended by a consultative council of Mayors/Do Governors prescribed in Article 165 of the Local Autonomy Act;
- Persons recommended by a non-profit, non-governmental organization defined in Article 2 of the Assistance for Non-Profit, Non-Governmental Organizations Act;
- Persons with considerable knowledge and experience in infectious diseases.
Holders of public offices must be in the minority.
The Committee has the ability to establish Advisory Committees to improve efficiency. One of these Advisory Committees is the Advisory Committee on vaccine compensation, who advise the minister on vaccine injury compensation claims. To assist the Vaccine Injury Committee Vaccine Injury Investigation Teams are appointed by the Director of KDCA from public officials working for KDCA, see Article 21 of the Decree. Vaccine Injury investigation teams consist of no more than ten people who are either experts in vaccination and adverse vaccine reactions or medical personnel. The Vaccine injury Investigation Team considers:-
- the report submitted by the Mayor/Do Governor,
- whether the 'injury is caused by vaccination… …regardless of abnormality of the relevant vaccine, or negligence of the person who performed the vaccination' Article 71(2) of the Act, and
- other matters determined by the Advisory Committee on Vaccination Injury Compensation in relation to vaccination injury compensation
The finding of the Vaccine Injury Investigation Team are used by the Vaccine Injury Compensation Committee. In Table 3 of their 2021 Article Ro et al set out the Criteria for Causal relationship review by the Vaccine Injury Compensation Committee as follows:-
| 1. Relevance The obvious case (definitely related, definite) | If there is clear evidence of vaccination, there is proximity to the chronological sequence in which abnormal reactions have emerged, and, for any other reason, causality by vaccination is recognized, and it is recognized as a known vaccine response. |
| 2. If there is a possibility of relevance (probably related, probable) | If there is clear evidence of vaccination, there is proximity to the chronological sequence in which abnormal reactions have emerged, and the causality of the vaccine is recognized more than for any other reason. |
| 3. If there is a possibility of relevance (possibly related, possible) | If there is clear evidence of vaccination and there is proximity to the chronological order in which abnormal reactions have emerged, but the occurrence of results for other reasons is also recognized as the same level of probability as vaccination. |
| 4. Relevant to be acknowledged difficult cases (probably not related, unlikely) | In the case that there is clear evidence of vaccination, and the time sequence in which the abnormal reaction occurred is less close, and the possibility of the vaccine is unclear. |
| 5. Clear the relevance is not the case (definitely not related) | If there is no clear evidence of vaccination, if there is no close proximity of the chronological order in which the adverse reaction has emerged, or if any other obvious cause has been identified. |
Appeals and the right to litigate
Use of the scheme and litigation are mutually exclusive and a claimant must choose which one they take. If an individual litigates against the manufacturer, vaccine administrator, etc and succeeds they cannot claim from the NFCS and any moneys already paid out by the NFCS will be recovered.
There is an external review process where the Courts review how the decision was made. If the claim to the NFCS is rejected the claimant can claim against the Head of the KDCA, the usual litigation rules apply, the proof of causation is on the claimant. From the judgment in Seoul Administrative Court. Sentencing 2018 Guhap78619 (November 13, 2019) it appears that the court will only examine whether there was abuse of discretion on the part of the head of KDCA, not the substance of the decision.
Useful information and links
The Provisions creating the NFCS are set out in legislation, see below. We have not been able to find any further information on the Compensation & Support Center for Covid-19 Vaccine Injury, who are reported to be the scheme administrators.
Public Bodies
Korea Disease Control and Prevention Agency - KDCA
Legislation.
Article 71 of the Infectious Disease Control and Prevention Act (Act No. 17067, March 4 2020)
Enforcement Decree of the Infections Disease Control and Prevention Act (Presidential Decree No. 28070, May 29 2017)).
Academic Articles
Ro D, Ro D, Kim SY. COVID-19 vaccine injury compensation programs. J Glob Health Sci. 2021 Dec;3(2):e21. https://doi.org/10.35500/jghs.2021.3.e21
News Reports & Blogs
Seo Ji-Eun & Yi Woo-Lim (2022) ‘Centre takes charge of vaccine side effect cases’ Korean JoongAng Daily 19 July 2022 available at https://koreajoongangdaily.joins.com/2022/07/19/national/socialAffairs/Korea-Covid19-vaccine/20220719183804365.html (Accessed 1 March 2023)
Joong Ang Ilbo (2022) ‘South Korea Opens Covid Vaccine Compensation Side Effect Centre’ RokDrop 20 July 2022 [Blog] available at https://www.rokdrop.net/2022/07/20/south-korea-opens-covid-vaccine-side-effects-compensation-center/ (Accessed 1 March 2023)
